| Home Page | Overview | Site Map | Index | Appendix | Illustration | FAQ | About | Contact |
 |
 |
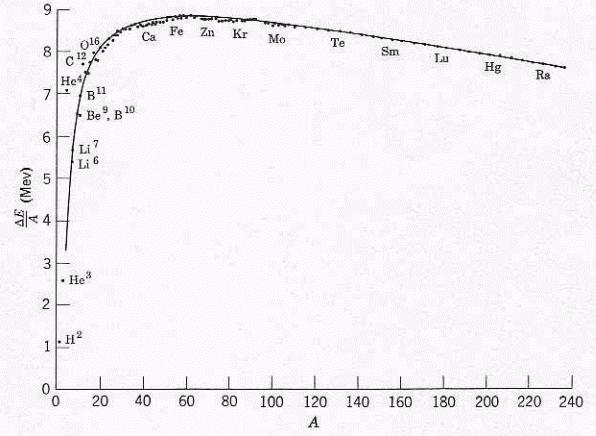
A nucleus is specified by its number of protons Z, number of neutrons N, and the mass number A = Z+N. The nucleons (protons and neutrons) in a nucleus are bound together -- their total energy is less than the total energy of the separated particles. The binding energy is the amount of energy given up when the nucleus is formed. Plotting the binding energy per nucleon versus the mass number A (Figure 14-01) shows that starting from Hydrogen, nuclei become more |
Figure 14-01 Nuclear Binding Energy |
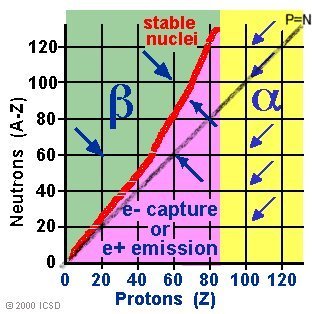
Figure 14-02 Proton/Neutron & Decay [view large image] |
stable as there are more protons and neutrons, until Iron. After that, the trend reverses. |
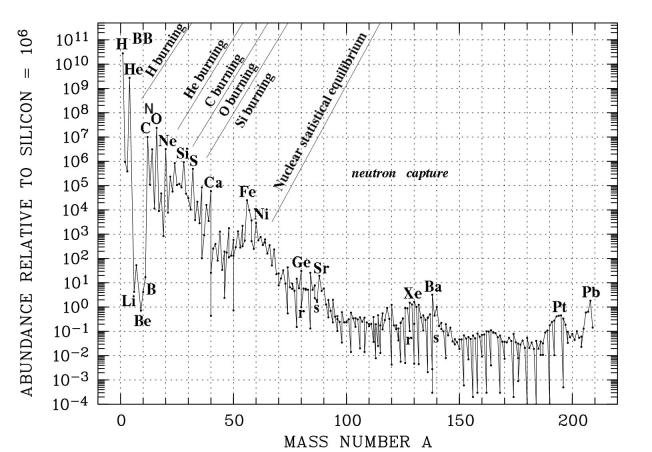 |
Light elements (mainly hydrogen, helium and trace of deuterium, lithium) were generated in the first few minutes of the Big Bang, which was not able to produce more complex elements as the universe rapidly cooling off. Since then hydrogen and helium contribute by mass of respectively 70 and 28 per cent of all baryonic matter in the universe. Most of the remaining 2% of the elements up to iron and nickel are made in the interior of the stars. The resulting elements are thrust into space by booming stellar winds or when a star explodes as a supernova. Carbon, nitrogen and oxygen are the most abundant heavy elements. Oxygen is created by supernovae, while carbon is created in low-mass stars (red giants, planetary nebulae) and nitrogen is by a mixture of the above. |
Figure 14-03 Element Abundance [view large |
image] |
 |
 |
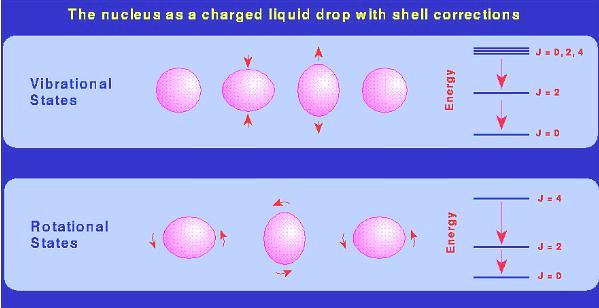
The liquid-drop model assumes that the constituents of the nucleus interact only with their nearest neighbors, and the density is constant inside the nucleus, like the molecules in the liquid. Using this analogy, a semiempirical formula has been developed to describe the binding energy as a function of the mass number A (shown by the solid curve in Figure 14-01). The result is not particularly accurate for the lower value of A. The expression is useful in discussing stability, radioactivity, and the fluctuations from the average behavior due to shell effects. The top diagram in Figure 14-04a shows two vibrational energy levels, which split into finer structures due to rotation. |
Figure 14-04a Liquid Drop Model [view large image] |
Figure 14-04b Fission [view large image] |
Figure 14-04b shows the deformation of the liquid drop, which eventually separates into two pieces (caused by the electrostatic repulsion of the protons). |
 |
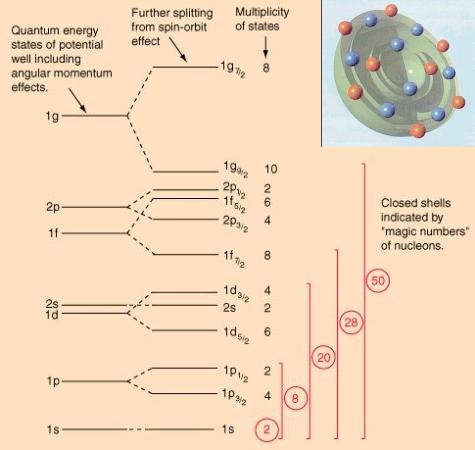 |
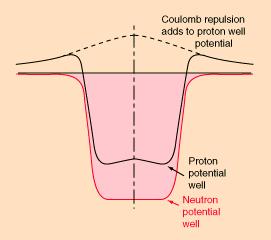
There is extensive experimental evidence of the contrary hypothesis that the nucleons move in an effective potential well created by all the other nucleons. Since the nucleons are densely packed into a small region, it is expected that the chance of collision is very high. However, the interaction by collision is minimized by the Pauli exclusion principle, which forbids two fermions to occupy the same quantum state. If there are no nearby, unfilled quantum states that can be reached by the available energy for an interaction, then the interaction will not occur. |
Figure 14-05a Nuclear Potential |
Figure 14-05b Nuclear Energy Levels [view large image] |
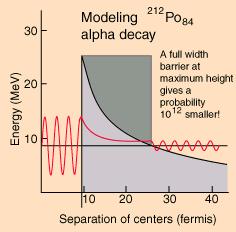
Unstable nuclei, called radioactive isotopes, will undergo nuclear decay to make it more stable. There are only certain types of nuclear decay which means that most isotopes can't jump directly from being unstable to being stable. It often takes several decays to eventually become a stable nucleus. When unstable nuclei decay, the reactions generally involve the emission of a particle and or energy. Half-lives are characteristic properties of the various unstable atomic nuclei and the particular way in which they decay. Alpha and beta decay are generally slower processes than gamma decay. Half-lives for beta decay range upward from 10-2 sec and, for alpha decay, upward from about 10-6 sec. Bismuth-209 has the longest half-life of 2x1019 years. Half-lives for gamma decay may be too short to measure (~ 10-14 second), though a wide range of half-lives for gamma emission has been reported.
 |
|
Figure 14-06 Alpha Decay |
through the Coulomb barrier; the nucleus has a small probability of escape to the outside depending on the height and width of the wall. |
 |
|
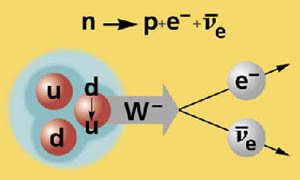
Figure 14-07 Beta Decay |
shows that in the beta decay process, the down quark turns into an up quark (thus changes the neutron to proton) by emitting a W- meson, which decays into an electron and an electron-anti-neutrino. |
 |
|
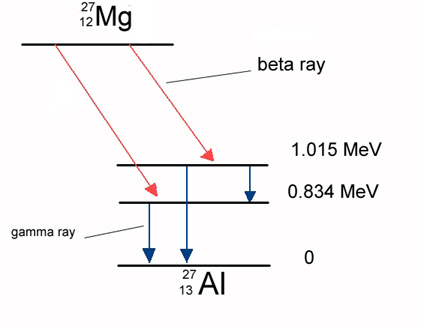
Figure 14-08 Gamma Decay |
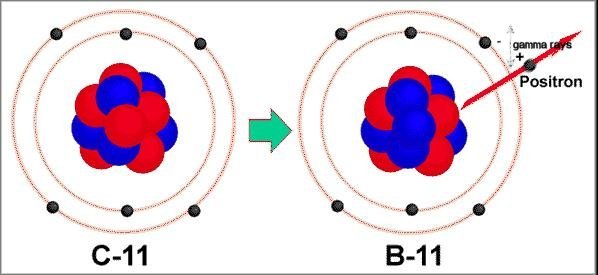 |
the energy contained in their mass appears as two gamma-rays of 0.5 MeV each, flying off in opposite directions. Positron radioactivity is therefore always accompanied by the emission of gamma rays with an energy of about 0.5 MeV in addition to any other gamma-rays which might be emitted. Example isotopes, which emit positrons are C-11, N-13, O-15 and F-18. These isotopes are used in positron emission tomography (PET). Figure 14-09 shows the transmutation of C-11 into B-11 by positron emission. |
Figure 14-09 Positron Emission |
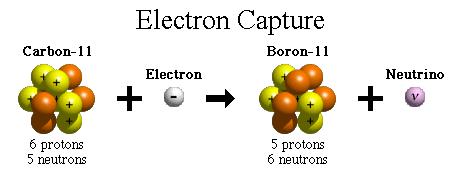 |
number of neutrons increases by 1, the number of protons decreases by 1, and the atomic mass remains unchanged. By changing the number of protons, electron capture transforms the nucleus into a new element. Electron capture is also called K-capture since the captured electron usually comes from the atom's K-shell. Figure 14-10 shows another way of transmuting C-11 into B-11 by electron capture. |
Figure 14-10 Electron Capture |
| Type | Emission | Penetrating Power | Example |
|---|---|---|---|
| Alpha Decay | Helium nuclei | 1, stopped by skin, very damaging due to ionization | 92U238 => 90Th234 + 2He4 Applicable to nuclei with Z>83, see Figure 14-02 |
| Beta Decay | Electron, high speed | 100, penetrates human tissue to ~ 1 cm | 53I131 => 54Xe131 + -1e0 Applicable to nuclei with high neutron-proton ratio |
| Gamma Decay | Photons, high energy | 10000, highly penetrating but not very ionizing | 92U238 => 90Th234 + 2He4 + 2 photon Energy lost from settling within the nucleus after transmutation |
| Positron Emission | Positron | 100 | 6C11 => 5B11 + 1e0 Applicable to nuclei with a low neutron-proton ratio |
| Electron Capture | Electron, inner shell | Neutrino | 37Rb81 + -1e0 => 36Kr81 + neutrino Applicable to nuclei with a low neutron-proton ratio |
 |
 |
If a massive nucleus like uranium-235 breaks apart (fission), then there will be a net yield of energy because the sum of the masses of the fragments will be less than the mass of the uranium nucleus. If the mass of the fragments is equal to or greater than that of iron at the peak of the binding energy curve, |
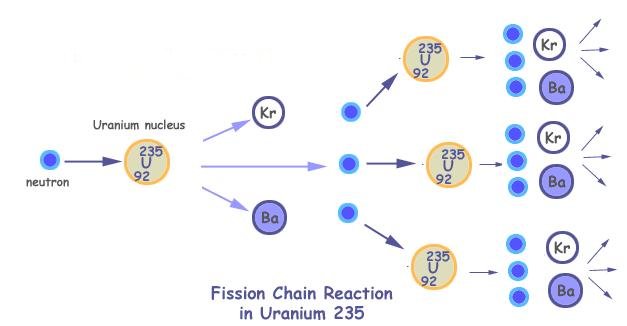
Figure 14-11 Chain Reaction |
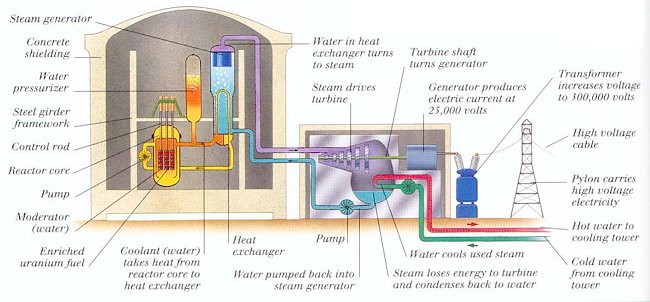
Figure 14-12 Nuclear Reactor |
(see Figure 14-01) then the products of the decay will be more tightly bound than they were in the uranium nucleus, and that decrease in mass comes off |
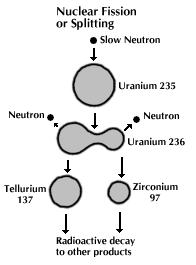
In one of the most remarkable phenomena in nature, a neutron can be captured by a uranium-235 nucleus, rendering it unstable toward nuclear fission. When a U-235 nucleus splits, it gives off energy in the form of heat and Gamma radiation, which is the most powerful form of radioactivity and the most lethal. When this reaction occurs, the split nucleus will also give off two or three of its `spare' neutrons. These spare neutrons fly out to split other U-235 nuclei they come in contact with. In theory, it is necessary to split only one U-235 nucleus, and the neutrons from this will split other U-235 nuclei, which will split more...so on and so forth -- a chain reaction. (See Figure 14-11.) This progression does not take place arithmetically, but geometrically. All of this will happen within a millionth of a second. The minimum amount to start a chain reaction as described above is known as Critical Mass. The actual mass needed to facilitate this chain reaction depends upon the purity of the material and whether it is tampered. The tamper is a thick casing made of natural uranium. It reflected neutrons back into the core and helped to hold it together for a fraction of a second. For pure U-235, the bare critical mass is 50 kg and the tampered critical mass is 15 kg (with a spherical tamper size of 11.5 cm diameter); it is 10 kg and 5 kg respectively for Pu-239 (with 8 cm tamper size).
While uranium-235 is the naturally occurring fissionable isotope, there are other isotopes which can be induced to fission by neutron bombardment. Plutonium-239 is also fissionable, and both types have been used to make nuclear fission bombs. Plutonium-239 can be produced by breeding it from non-fissionable uranium-238 (by absorbing a neutron and then transmuted via the beta decay process). Spent fuel is taken out of the reactor after four years and can be recycled in a reprocessing plant. Some of the nuclear reactors at Hanford, Washington and the Savannah-River Plant, SC are designed for the production of bomb-grade plutonium-239. The other isotopes known to undergo fission upon neutron bombardment are listed in Table 14-02, which displays the critical (bare) mass, half-life, number of neutrons generated in spontaneous fission, and the rate of heat generation by radioactive decay. They all undergo transmutation via the alpha decay process. For comparison, the table also includes the two major fertile materials, Thorium-232 and Uranium-238, which in the presence of neutrons can produce the fissionable isotopes Uranium-233 and Plutonium-239, respectively.| Fissionable Isotope | Crtiical Mass (kg) | Half Life (years) | Neutron Generation (# / sec-kg) |
Power Generation (Watts / kg) |
|---|---|---|---|---|
| Protactinium-231 | 162 | 3.28x104 | nil | 1.3 |
| Thorium-232 | Infinite | 1.41x1010 | nil | nil |
| Uranium-233 | 16.4 | 1.59x105 | 1.23 | 0.281 |
| Uranium-235 | 47.9 | 7.0x108 | 0.364 | 6x10-5 |
| Uranium-238 | Infinite | 4.5x109 | 0.11 | 8x10-6 |
| Neptunium-237 | 59 | 2.14x106 | 0.139 | 0.021 |
| Plutonium-238 | 10 | 88 | 2.67x106 | 560 |
| Plutonium-239 | 10.2 | 2.41x104 | 21.8 | 2.0 |
| Plutonium-240 | 36.8 | 6.54x103 | 1.03x106 | 7.0 |
| Plutonium-241 | 12.9 | 14.7 | 49.3 | 6.4 |
| Plutonium-242 | 89 | 3.76x105 | 1.73x106 | 0.12 |
| Americium-241 | 57 | 433 | 1540 | 115 |
| Americium-242 | 9 - 18 | - | - | - |
| Americium-243 | 155 | 7.38x103 | 900 | 6.4 |
| Curium-244 | 28 | 18.1 | 1.1x1010 | 2.8x103 |
| Curium-245 | 13 | 8.5x103 | 1.47x105 | 5.7 |
| Curium-246 | 84 | 4.7x103 | 9x109 | 10 |
| Curium-247 | 7 | 1.55x107 | - | - |
| Berkelium-247 | 10 | 1.4x103 | nil | 36 |
| Californium-251 | 9 | 898 | nil | 56 |
 |
 |
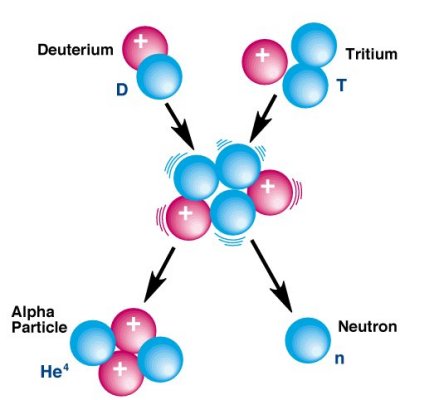
Thermonuclear, or hydrogen bombs explode with enormous power via uncontrolled self-sustaining chain fusion reactions. Deuterium and tritium form helium under extremely high temperature, providing the energy:
D + T ==> He + n + 17.6 Mev (See Figure 14-13.) In principle, a mixture of D and T heated to very high temperature and in high density will start a chain fusion |
Figure 14-13 Fusion |
Figure 14-14 Thermalnuclear |
reaction, liberating an enormous amount of energy. But tritium is an unstable element; an ingenious method is to have it produced from lithium deuterate (Li6H2) in |
|
the fission phase of the explosion -- thus one compound is used for both types of fuels (D and T). In a thermonuclear bomb, the explosive process begins with the detonation of what is called the primary stage. This consists of a relatively small quantity of conventional explosives, its detonation brings together enough fissionable uranium to create a fission chain reaction, which in turn produces another fission explosion inside the temper and raises the temperature to several million degrees. When the temperature of the mixture reaches 10,000,000 oK, fusion reactions take place. The neutrons from the fusion reactions induced fission in the uranium-238 pieces from the tamper and shield, which produced even more radiation and heat and the bomb exploded. (See Figure 14-14.)
Specialized type of small fusion bombs designed to release | neutrons rather than causing further fission reactions are called neutron bombs. This is accomplished by removing the U-238 tamper. Neutrons kill people, leaving the hardware and buildings intact. It is a "clean" bomb.
The theorized cobalt bomb is, on the contrary, a radioactively "dirty" bomb having a cobalt tamper. This tamper is made of cobalt-59, which is transmuted into cobalt-60 by neutrons released from the fusion reactions. Cobalt-60 has a half-life of 5.26 years and produces energetic (and thus penetrating) gamma rays. The half-life of Cobalt-60 is just long enough so that airborne particles will settle and coat the earth's surface before significant decay has occurred, thus making it impractical to hide in shelters. This is the "doomsday machine" since it is capable of wiping out life on earth. |
 |
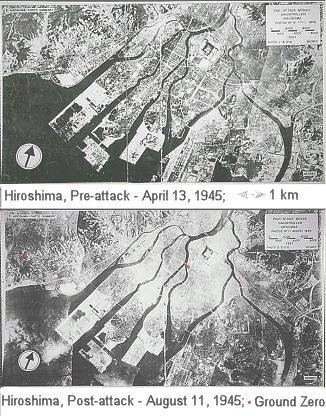 |
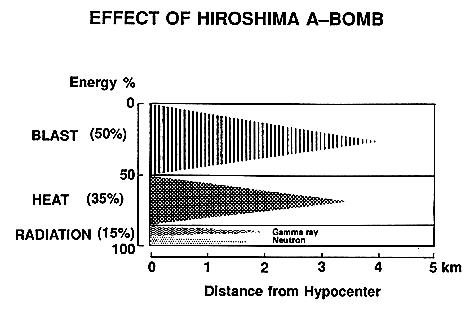
At the hypocenter (ground zero), everything is immediately vaporized by the high temperature (up to 300 million oC). Outward from the hypocenter, most casualties are caused by burns from the heat, injuries from the flying debris of buildings collapsed by the shock wave, and acute exposure to the high radiation. Beyond the immediate blast area, casualties are caused from the heat, radiation, and fires spawned from the heat wave. Figure 14-16 presents two views of Hiroshima before and after an atomic-bomb attack. It occurred in the morning (8:16 a.m.) of August 6, 1945. |
Figure 14-15 Effects of A-Bomb [view large image] |
Figure 14-16 Hiroshima |
The bomb detonated at an altitude of 580 meters killing or wounding about half of its 350,000 inhabitants with long-term effects on incalculable numbers among the survivors. |