Atoms are electrically neutral, and the positiveness of the protons is balanced by the negativeness of the electrons. It follows that in a neutral atom:
no of electrons = no of protons
So, if an oxygen atom (atomic number = 8) has 8 protons, it must also have 8 electrons; if a chlorine atom (atomic number = 17) has 17 protons, it must also have 17 electrons.
The arrangement of the electrons
The electrons are found at considerable distances from the nucleus in a series of levels called energy levels. Each energy level can only hold a certain number of electrons.
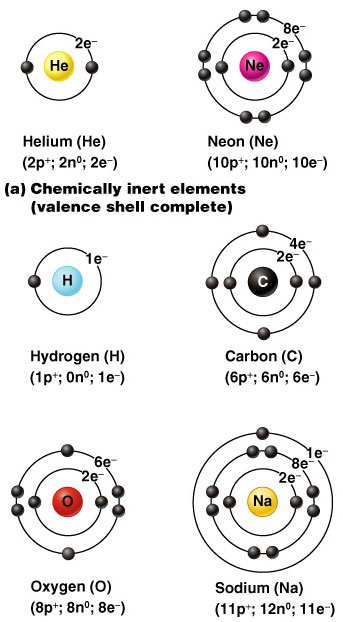
The first level (nearest the nucleus) will only hold 2 electrons, the second holds 8, and the third also seems to be full when it has 8 electrons. At GCSE you stop there because the pattern gets more complicated after that.
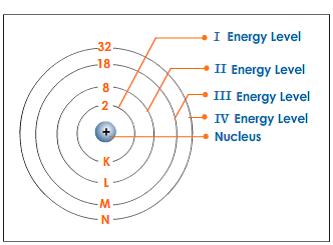
These levels can be thought of as getting progressively further from the nucleus. Electrons will always go into the lowest possible energy level that is nearest to the nucleus if there is space.
 ශිල්ප 64
ශිල්ප 64