Isotopes are atoms which have the same atomic number but different mass numbers. They have the same number of protons but different numbers of neutrons. The number of neutrons in an atom can vary within small limits. For example, there are three kinds of carbon atom 12C, 13C and 14C. They all have the same number of protons, but the number of neutrons varies.
| protons | neutrons | mass number | |
| carbon-12 | 6 | 6 | 12 |
| carbon-13 | 6 | 7 | 13 |
| carbon-14 | 6 | 8 | 14 |
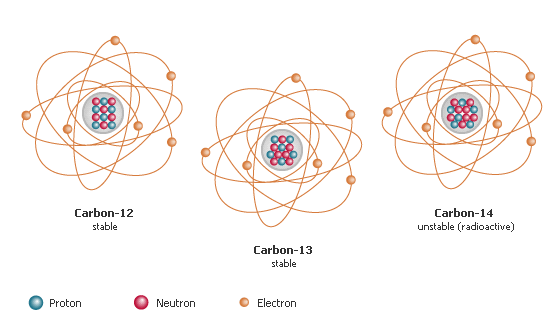
These different atoms of carbon are called isotopes. The varying numbers of neutrons makes no difference in chemical reactions.
 ශිල්ප 64
ශිල්ප 64