The atom is a basic unit of matter. It consists of a dense central nucleus surrounded by a cloud of electrons, protons and neutrons.
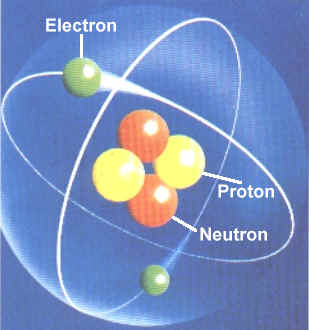
The electrons are negatively charged, protons are positively charged while neutrons are nuetral. Nuetrons are bound to the nucleus by the electromagnetic force.
An atom containing an equal number of protons and electrons is electrically neutral, otherwise it has a positive charge or negative charge. An atom is classified according to the number of protons and neutrons in its nucleus.
The number of protons determines the chemical element.
The No of protons = ATOMIC NUMBER of the atom.
The number of neutrons determines the isotope of the element.
 ශිල්ප 64
ශිල්ප 64