Carbon nanotubes have been constructed with length-to-diameter ratio of up to 132,000,000:1. These cylindrical carbon molecules are used in many applications in nanotechnology, electronics, optics, and other fields of materials science and architecture fields.
Carbon nanotubes used to construct body armor with extraordinary strength and quite less weight. Carbon nanotubes also have unique electrical properties and efficient thermal conductors.
The diameter of a nanotube is few nanometers (approximately 1/50,000 of a human hair. Nanotubes are constructuted as single-walled(SWNTs) and multi-walled (MWNTs).
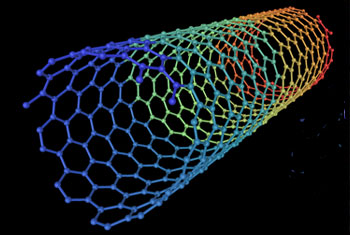
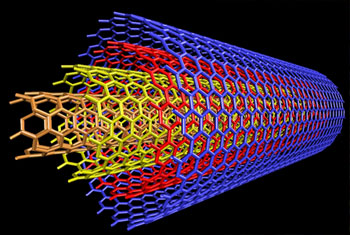
Quantum chemistry describes chemical bonding in nanotubes. The chemical bonding of nanotubes is composed entirely of sp2 bonds, similar to those of graphite.
These bonds, which are stronger than the sp3 bonds found in alkanes, provide nanotubues unique strength. Nanotubes naturally align themselves into "ropes" held together by van der Waals forces.
Carbon nanotubes used to construct body armor with extraordinary strength and quite less weight. Carbon nanotubes also have unique electrical properties and efficient thermal conductors.
The diameter of a nanotube is few nanometers (approximately 1/50,000 of a human hair. Nanotubes are constructuted as single-walled(SWNTs) and multi-walled (MWNTs).


Quantum chemistry describes chemical bonding in nanotubes. The chemical bonding of nanotubes is composed entirely of sp2 bonds, similar to those of graphite.
These bonds, which are stronger than the sp3 bonds found in alkanes, provide nanotubues unique strength. Nanotubes naturally align themselves into "ropes" held together by van der Waals forces.
 ශිල්ප 64
ශිල්ප 64