
- Choose the most appropriate (reasonably
close) answer...
- The radius of a (12 oz) soda can is
about:
- 1.2 mm
- 1.2 ft
|
- 1.2 cm
- 1.2 in
|
- The Circumference of an average-sized lemon would be
about:
- The volume of a small aquarium would be about:
- 1 qt
- 10 gal
|
- 100 mL
- 10,000 L
|
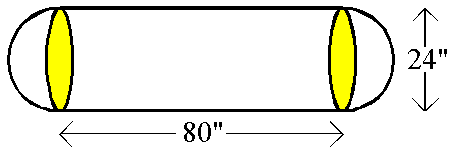
- An LPG (liquified propane gas) tank is shown in the
illustration below. How many gallons of
propane could this tank hold?
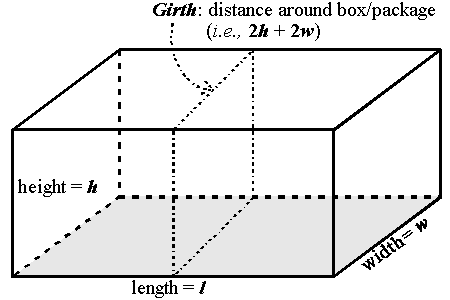
- The U.S. Postal Service has certain requirements
regarding the size of packages to be mailed. The
girth (see illustration below) plus the length
cannot exceed 108 inches. If a package/box
is to have maximum volume and minimum surface area, it must
be a cube (i.e., l = w = h).
- For the largest such possible package/box, show that
the measure of any side will be 21.6 inches.
- Find the volume (in cubic feet) of
the package/box which meets these requirements.
- What is the surface area of this same package/box in
square feet?
-
 The [1979] 280-Z automobile, built by Datsun (now Nissan),
has a 6-cylinder engine where the "bore × stroke"
of each cylinder is "8.6 cm × 7.9
cm." Show that the car has an
engine size (a.k.a. displacement) of about
2800 cc, when rounded to the nearest hundred.
The [1979] 280-Z automobile, built by Datsun (now Nissan),
has a 6-cylinder engine where the "bore × stroke"
of each cylinder is "8.6 cm × 7.9
cm." Show that the car has an
engine size (a.k.a. displacement) of about
2800 cc, when rounded to the nearest hundred.
- The [1994] Italian made Ferrari 456GT automobile has a
V-12 engine where each cylinder was designed with a
"bore × stroke" of "88 mm
× 75 mm." Find the car's engine size
(a.k.a. displacement)
 when measured in:
when measured in:
- cubic centimeters (cc)
- liters (L)
- cubic inches (in3)
|
- Density (ρ) is defined to be the
mass (or its weight) divided by its volume (i.e.,
ρ = m/v).

| a. |
The density of water (H2O)
is 1.00 g/cc.
How many pounds does one
gallon of water weigh?
|
| b. |
 The density of lead (Pb) is 11.35 g/cc.
How many pounds would a
gallon of molten lead weigh?
The density of lead (Pb) is 11.35 g/cc.
How many pounds would a
gallon of molten lead weigh?
|
 A twin-size water bed has a water-filled mattress,
rectangular in shape, which has dimensions of 6 ft
(length) by 4 ft (wide) by 6 in
(thick). How much would this mattress weigh, when full, in
pounds? Hint: Use a result from the
previous problem (see #6a, above).
A twin-size water bed has a water-filled mattress,
rectangular in shape, which has dimensions of 6 ft
(length) by 4 ft (wide) by 6 in
(thick). How much would this mattress weigh, when full, in
pounds? Hint: Use a result from the
previous problem (see #6a, above).
- A cylindrical water tank holds 8000 gallons
and it has a
 height = 50 3/8",
find the:
height = 50 3/8",
find the:
- volume/capacity of the tank in ft3.
- diameter of the tank in feet.
- minimum-sized cover, to fit the top of the tank, in
ft2.
- cover size needed if it must over-hang the top rim (i.e.,
circumference) of the tank by 1 ft.

- A refrigerator has two (rectangular box) compartments,
side by side. One side has interior dimensions of being 16"
wide, 25" deep, and 57.5" high;
while the freezer has interior dimensions of being 10"
wide, 25" deep, and 57.5" high
(also). What is the capacity (volume) of the entire
refrigerator in cubic feet?
- Humans breathe with a resting rate of about 15 breaths
per minute and each breath utilizes about 250 mL
of oxygen (O2). Air is
generally 78% nitrogen (N2) and
21% O2. If you were trapped in
a sealed rectangular space whose dimensions are 2
meters by 5 meters by 10
meters, then how long until the oxygen supply is
completely consumed?
- Realistically, a person becomes subject to suffocation
once the carbon dioxide (CO2)
levels reach the 4% level, and normal breathing typically
results in 50% of the O2
inhaled being respired as CO2.
In the same confined space and with the same breathing rate
as described in the previous problem (#10 above), how long
until the CO2 concentration
reaches a dangerous level?
- If you panic and/or are subject to strenuous exertion,
then your breathing rate could increase to ten times the
normal rate, given in problem #10 (above). Under such
circumstances,
- what would be your breathing rate in liters
per minute?
- If you were trapped in the same space as the
previous problems, #10 & #11 (above), then how long
might you expect to have until the CO2
begins to suffocate you?
|

